Bio-Assessment Program (NOW FREE ONLINE RESULTS & REPORTS!)
Progenitor stem cell and platelet biologics, stemming from whole blood, bone marrow and adipose tissue, are rapidly becoming a therapeutic standard of care for various conditions. It has become more evident that patient outcomes vary based on the product or technique used to prepare these cellular biologics.
The Bio-Assessment Program is a research tool developed for physicians and other clinical providers, and is dedicated to the independent review and assessment of products and processes used to prepare stem cell and platelet biologics. The Bio-Assessment Program has allocated bioassays that are relevant to clinical outcomes and consist of CBC with WBC differentiation, mesenchymal progenitor stem cell marker CFU-F, hematopoetic progenitor stem cell marker CD34+, and total nucleated cells TNC.
Clinicians can submit product samples, utilizing the Bioassay Kit, and track the results online through their private login. Performance results are automatically calculated and running averages are automatically maintained. These provide clinicians with qualified research data concerning the quality of their products and/or processes used to prepare their biologics. Performance reports are automatically generated outlining the overall performance of the practice. This report will provide patients with the assurance they need when making a decision on a regenerative biologic provider.
Track Your Data Online (for ongoing quality control)
Submit a sample and track your results Online for FREE. Online tracking of your results are free for 60 days. If you continue to submit samples within the 60 day time period, your online service will remain active without interruption. Track your data and take advantage of the critical calculations that determines the ongoing effectiveness of your biologics program. Get the data you need to give your patients the confidence of quality.
DEMO LOGIN
Username: testuser
Password: test0000
The Bio-Assessment Program is Comprehensive
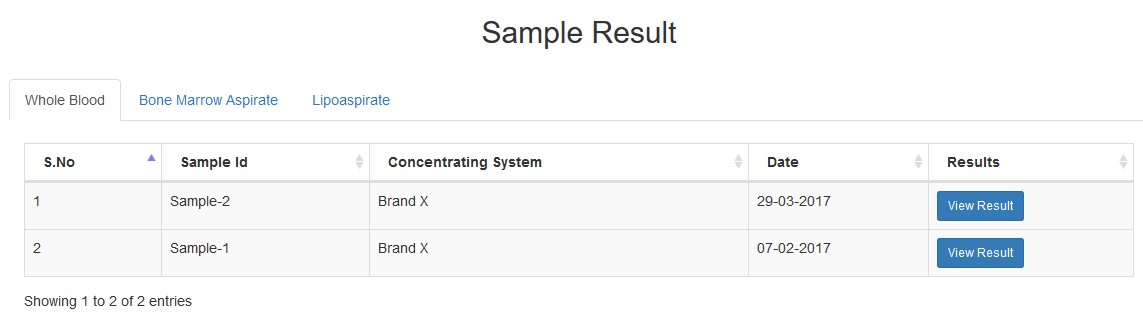 |
Keep a running tab of your PRP, BMA & Lipoaspirate sample results
|
|
|
|
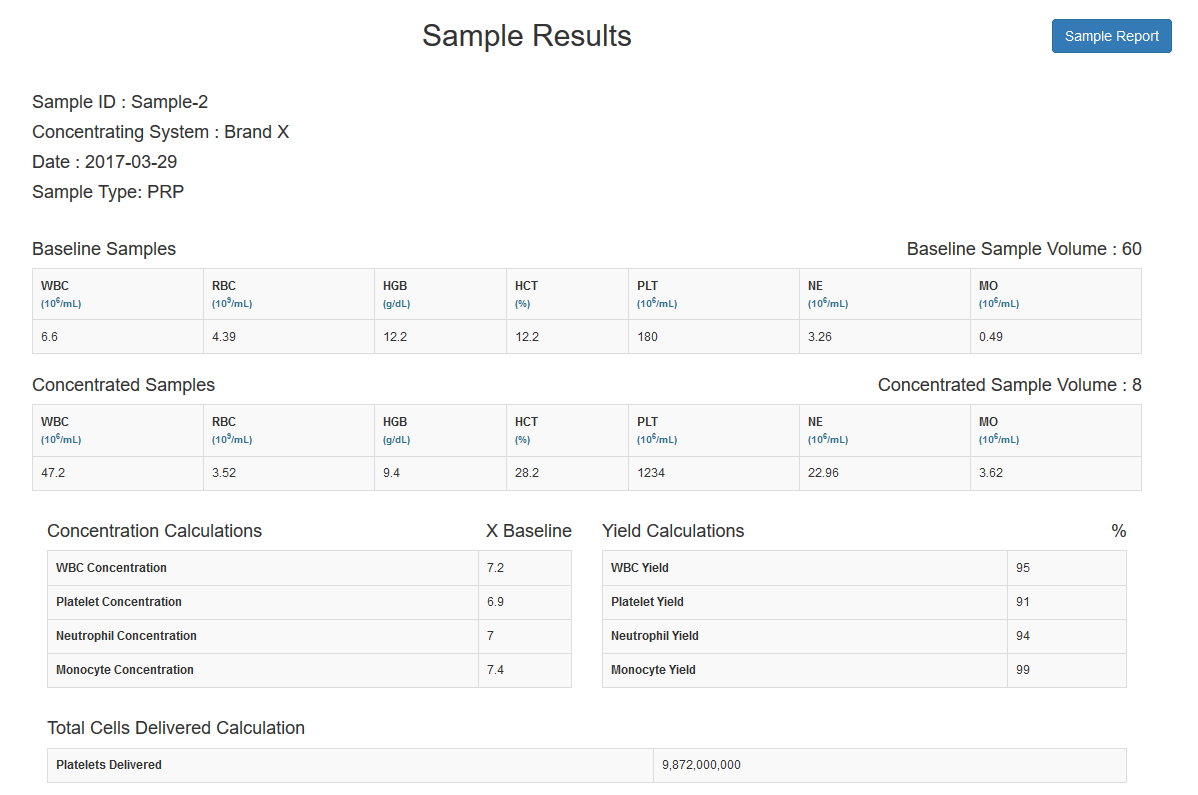
Review individual sample results including Concentration and Yield calculations |
|
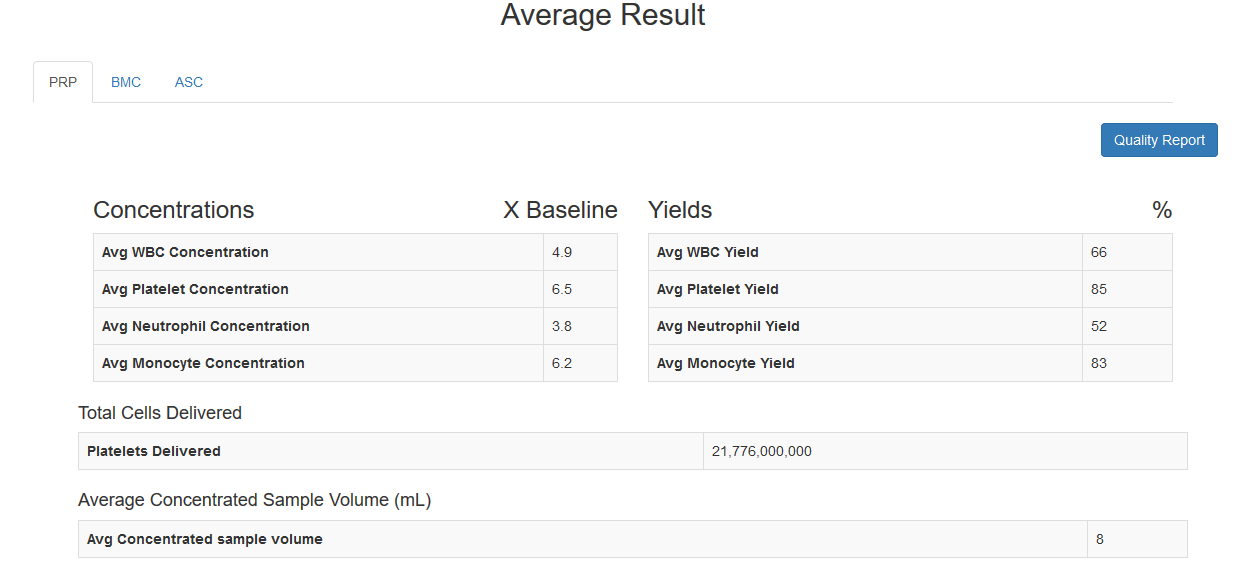 |
Track the overall performance of your facility with automatically calculated Running Averages for all sample types |
How Does the Bio-Assessment Program Work
The following steps will take you through the process.
- Configure your Bioassay Kit
- Prepare and ship your sample to BSR.
- Login to track your results online.
IMPORTANT REMINDERS:
- Bioassay samples MUST be shipped from Monday to Thursday. No samples can be shipped on Friday.
BSR Certificate Program
The BSR Certificate represents a high standard of care in regenerative biologics. To attain this certificate, your practice must pass the biologics performance criteria. This certificate is the first step towards standardization of care in biologics and a representation of defined treatment in biologics. If you believe your practice can pass the performance criteria, then enroll for the evaluation. ENROLL HERE
BSR PRP CERTIFICATE
Criteria requires that ten (10) consecutive samples of the same approximate volume. Each sample result requirement:
- Platelet yield ≥ 75%
- Platelet concentration ≥ 5 times baseline
- Deliverable platelets ≥ 1 billion per mL
BSR BMA CERTIFICATE
Criteria requires that five (5) consecutive samples of the same approximate volume. Sample result requirement:
- CFU-F yield ≥ 70%
- CFU-F concentration ≥ 4 times baseline
- CD34+ yield ≥ 70%
- CD34+ concentration ≥ 4 times baseline
- TNC yield ≥ 70%
- TNC concentration ≥ 4 times baseline
IMPORTANT REMINDERS:
- Certificates are valid for two (2) years.
- A complete report is provided at the end of the evaluation.
- A certificate will be provided ONLY for successful evaluations.
- Evaluations are NOT refundable.