Assay Validation

 BSR will qualify commercial immunoassay kits when available or develop assays when one is not available for your special requirements. BSR has extensive expertise in developing, optimizing and validating Immunoassays for Research & Development, Manufacture and Clinical research (Preclinical through Phase IV clinical studies). All studies are under BSR's Quality Systems and are GLP and cGMP compliant.
BSR will qualify commercial immunoassay kits when available or develop assays when one is not available for your special requirements. BSR has extensive expertise in developing, optimizing and validating Immunoassays for Research & Development, Manufacture and Clinical research (Preclinical through Phase IV clinical studies). All studies are under BSR's Quality Systems and are GLP and cGMP compliant.
BSR has been successful with immunoassay development for the following Antigen Families: Cytokines, Chemokines and Growth Factors, Kinases, Transcription factors, Adhesion molecules, MMP's, BMP's, Peptide and Protein Drugs, Drug related antibodies, and Autoantibody. Immunoassay development can support research areas of: Apoptosis, Endocrinology, Immunology, Signal Transduction, Stem Cell development, Cancer Biology, Development and Hematology. Methods include: ELISA, Multiplex array, Flow cytometry, Western Blot, Immunoelectrophoresis and Immunodiffusion.
Validation:
Compendial assays or Commercial assays with sufficient documentation on performance and acceptance criteria are employed by BSR after qualification for sample matrix and any special conditions for the intended application. Other assays are validated according to best industry standards including: FDA “ Guidance for Industry: Bioanalytical Method Validation” CDER 2001; and ICH Q2(R1) “Validation of Analytical Procedures: Text and Methodology”.
Click Here for Assay Validation Services
Bioassays:
BSR is experienced in developing and validating bioassays that measure the response of cells in culture or in vivo. Bioassays present different challenges than ligand binding assays. Rather than a focus on concentration or concentration related variables (e.g. mg/ml, IC50 or EC50), Bioassays return a relative potency. This approach can minimize the large day-to-day test variation attributed to test cells.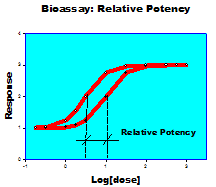
BSR can deliver service for Bioassay development and validation services for product potency documentation or demonstration of efficacy in clinical trial research.
Bioassays developed by BSR include: Kinase Phosphorylation (e.g. SMAD, Akt, PI3, HER2/ErbB2, Erk1/2), Cell Differentiation (Mesenchymal: Osteogenic, Adipogenic and Chondrogenic; Hematopoietic Stem cells: CFU-E, BFU-E, CFU-GEMM, CFU-GM, CFU-G, CFU-M). Cell Cycle Determination ( P21), Serum Complement (CH50), Platelet Function (aggregation, growth factor release, p-selectin expression, osmotic stress), Lymphocyte and Granulocyte Function (Stimulation, Cytotoxicity, Cell number, Viability, Apoptosis), Cell Migration (modified Boyden chamber, membrane and semisolid media) and Stem Cell Function (colony formation and differentiation).
Protein Function Assays:
BSR has developed an array of protein function assays to evaluate enzyme activity of Oxidoreductases, Transferases, Hydrolases, Lyases, Isomerases, and Ligases (EC1 – EC6). These assays are readily adapted to screen enzyme inhibitors. BSR has extensive experience with the enzymes and inhibitors of the serum complement and coagulation systems. BSR has developed methods to determine Collagen levels in tissue samples. Protein function can be determined by compendial methods or with assays developed and validated in-house.
Stem cell analysis:
 BSR develops protocols for stem cell analysis in blood, bone marrow and adipose tissues. Stem cell assays define and enumerate Stem cell and Progenitor populations by CD markers with cytometry or by function using growth and differentiation in defined media. CD34 positive Hematopoietic stem cells (HSC) form the progenitor cells: CFU-E, BFU-E, CFU-GEMM, CFU-GM, CFU-G, and CFU-M when grown in semi-solid media with appropriate growth factors. Mesenchymal cells (MSC) can be shown to differentiate toward: Osteogenic, Adipogenic, or Chondrogenic cells.
BSR develops protocols for stem cell analysis in blood, bone marrow and adipose tissues. Stem cell assays define and enumerate Stem cell and Progenitor populations by CD markers with cytometry or by function using growth and differentiation in defined media. CD34 positive Hematopoietic stem cells (HSC) form the progenitor cells: CFU-E, BFU-E, CFU-GEMM, CFU-GM, CFU-G, and CFU-M when grown in semi-solid media with appropriate growth factors. Mesenchymal cells (MSC) can be shown to differentiate toward: Osteogenic, Adipogenic, or Chondrogenic cells.